 by Dan Rusyniak
by Dan Rusyniak

When Gwyneth Paltrow “consciously uncoupled” from Chris Martin, I immediately thought of mitochondria. Why mitochondria? For one because mitochondria are the favorite organelle of toxicologists (sorry, sarcoplasmic reticulum . . .). And second, because I am fascinated by drugs that uncouple oxidative phosphorylation. There are lots of toxins that target mitochondria. There are drugs that inhibit mitochondrial DNA replication like the nucleotide nucleoside/nucleotide reverse transcriptase inhibitors (e.g., zidovudine).1 There are drugs that inhibit mitochondrial oxidative phosphorylation, like carbon monoxide2, hydrogen sulfide3, and of course, cyanide. But the ones I find the most interesting are toxins that uncouple oxidative phosphorylation, like dinitrophenol4 and aspirin (salicylate). These are uncouplers. I love them because nothing says toxicity like dissipating the membrane potential of the mitochondrial inner membrane space. What, you say? This is starting to hurt your head? Do you feel like you have been transported back to your first-year medical school lecture hall? OK, I promise to keep this light. In today’s post I will only focus on salicylate as an uncoupler and why maintaining a normal or alkaline serum pH is so critical in these cases.
To understand salicylate toxicity, we need to have a cursory understanding of uncoupling. And to do that you have to go back a bit, to biology class. You may recall that one of the main roles of the mitochondria is to synthesize ATP. It does this by creating a kind of battery inside your cells. Look at the diagram below. Electrons are donated from NADH (and for the really nerdy sometimes FADH2). These electrons then “flow” across the protein complexes (complex I, II, III, IV) creating a kind of “current”. It is important to note that for the current to flow it must go somewhere. In this case, that “current” (or moving electron) ultimately goes to oxygen. The main role of oxygen is to be the final electron acceptor in this process. It completes the circuit. The whole reason we breathe is so that this little electron has a final home, oxygen.
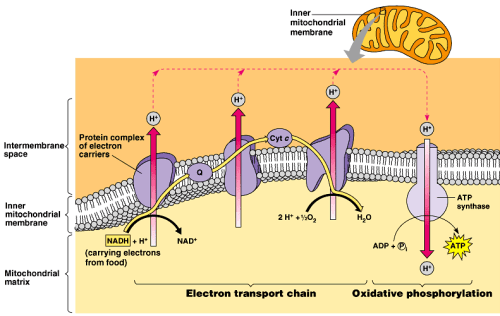
But I digress. So what do we do with the energy derived from this “current”? We use it to pump protons across a membrane. And what does moving protons across a membrane have to do with anything? Stay with me, we are almost done. Mitochondria are kind of weird. They have both an outer membrane and an inner membrane. Between those two (orange in the picture above) is the inner membrane space. This is where the protons end up. Think about it. When you move positive charges across a divide you end up with positive and negative (relatively speaking) charges separated across a potential space. Like I said above, this is like a battery, at least conceptually.

And like a battery, this charge separation represents potential energy which can be used to power things. In the case of the mitochondria, this energy is used to make ATP (the fuel all energy-dependent reactions run on). When it all works as designed, the process is said to be coupled. That is, electron flow is coupled to ATP formation.
So, what do uncouplers do? They uncouple electron flow from ATP formation. Salicylate is a great example of an uncoupler. It works by grabbing protons and shuttling them back across the inner mitochondrial membrane. It can also open up pores in the membrane but the effect is the same, it shorts out the battery. This causes less ATP formation. Now the body doesn’t take kindly to having less ATP. In response, it revs up the engine sending more and more electrons through the system. This results in big increases in metabolism, glycolysis, the TCA cycle, and all those processes that feed into this. So, what happens clinically when you rev up a system without producing more work? You get heat. This is why patients who overdose on uncouplers can get profoundly hyperthermic.5 This is akin to what happens if you sit in your car and floor the gas while the engine is in neutral. Lots of energy is consumed, but no work is produced – the engine overheats. Clinically this is also one of the ways that aspirin contributes to death. The lack of ATP targets the brain and the heart (they are so needy) causing the famous life-threatening dyad of a really bad aspirin overdose – cerebral and pulmonary edema.
So I promised I would talk about keeping pH normal and why that is important. First, it is important to mention why salicylate causes acidosis. We don’t know. OK, move on. To be fair we do know a lot about this, but it is complicated. Steve Curry and Meghan Spyres do a great job discussing this in their chapter on salicylate. The simple version is that under a normal state the hydrogen ions produced when ATP is consumed are balanced by the hydrogen ions used when ATP is made. As long as your metabolic demands are met, you are fine. But as I said above, at high concentrations salicylate is an uncoupler, so you are consuming energy faster than you can make it. This is one of the ways uncouplers cause metabolic acidosis. Acidosis is a really bad thing in large salicylate overdoses.
In toxicology, we usually don’t care much about a pH of 7.3. In a salicylate overdose, we do. This has to do with the dissociation constant of salicylate (first mitochondria and now pharmacokinetics . . . sorry). The dissociation constant is the pH at which half of the molecules in a solution are ionized and half are non-ionized. At a pH ABOVE the pKa, more of the molecules are ionized or charged and at a pH BELOW the pKA, more of the molecules are non-ionized, or uncharged. Things that are ionized, or charged, have a harder time crossing biologic membranes than those that are uncharged, or non-ionized. Acetylsalicylic acid is a weak acid (pKa=3.5, and salicylic acid has a pKa=2.97). This means at a normal serum pH of 7.4 the vast majority (~98%) of salicylate is negatively charged, or ionized. And that is a good thing because only unbound, non-ionized salicylate crosses membranes. Another way of saying this is so long as you keep the pH normal, most salicylate will stay out of the brain and heart. But remember (really, you don’t need to) pKa and pH are logarithmic. This means that even small changes in pH can have profound changes to the amount of ionized salicylate. If you drop serum pH from 7.4 to 7.2 you will almost double the amount of unionized salicylate that can go into the tissue. Protein binding and Michaelis Menten kinetics also matter (things that are protein bound also don’t cross membranes) but you have had enough pharmacology for today. So what does this all mean? It means to keep salicylate out of the heart and brain, where it causes its problems, you need to keep pH normal or even better, alkaline. In the sick patient with pH is < 7.4 start with boluses of sodium bicarbonate (1-2 meq/kg) followed by a bicarb drip. If the pH is normal, but levels are elevated (40 – 45 mg/dl) you just use a bicarb drip to try to alkalize the blood, and possibly the urine. To make a drip, most of us toxicologists will use three amps of sodium bicarb in 1L of D5W and run a drip at 1.5 – 2 times maintenance rate. On a side note, if you can also alkalize the urine then you get the added benefit of excreting more salicylate in the urine. Quick tip: to alkalinize the urine you must keep patients from getting hypokalemic6, cause the kidney will preferentially hold on to potassium and dump a proton, preventing the alkalinization. Keep their K > 4.0 meq/L.
Another way to maintain arterial pH is to breathe. As you likely recall, salicylates cause patients to hyperventilate. This causes the classic mixed acid-base disorder of respiratory alkalosis with metabolic acidosis (test tip: an ABG on a test with a low PCO2 and bicarb is probably a salicylate overdose). Why do salicylates cause people to breath deeper (hyperpnea) and faster (hyperventilation)? We don’t completely know. We know It is not from cyclooxygenase inhibition.7 We know it correlates with high metabolic rates.8 And, we know it involves the brain.9 Regardless of the reason, hyperventilation is a good thing. It helps to keep your serum pH normal to alkaline. And that is why you should never give drugs to blunt a sick aspirin overdose patient’s ventilatory rate. Why would you do that? Because the patient is sick. By sick, we are typically talking about acute overdoses with levels >80 mg/dl. As I have already discussed, at high concentrations salicylates uncouple oxidative phosphorylation. This means less ATP being formed in your brain. And what happens when your brain doesn’t make ATP? you get cerebral edema; salicylate poisoning may also lower brain glucose concentrations.10 Swollen, hangry brains in people who are uncoupling don’t work and patients get altered. What do we do with a hypotensive, altered, possibly hyperthermic patient with a pH < 7.2 in the emergency department? We intubate them. This is important, and the whole point of this post. We’re finally here.
If you are going to intubate a bad salicylate overdose you must keep up their minute ventilation. Aspirin patients pant in part because their survival depends on them keeping their serum pH alkaline. This is important to remember because many of the deaths that occur with aspirin overdose, do so shortly after they have been intubated. This is why published guidelines on managing salicylate poisoning say:
“. . . endotracheal intubation and mechanical ventilation can be associated with rapid worsening of clinical salicylate toxicity and increased mortality unless a normal or slightly alkalemic blood pH is maintained via hyperventilation.”
There is also some clinical and animal data to support this. In one retrospective study, ABGs that were obtained before and after intubation in aspirin overdoses were analyzed. All the patients post-intubation had significant reductions in pH and several of the patients who died deteriorated shortly after intubation.11 Anecdotally, at my poison center, we have seen similar problems. We recently had three aspirin deaths reported to our center. All of them occurred shortly after the patient was intubated. By now this should all make sense.
- At high concentrations salicylate uncouples oxidative phosphorylation
- Uncoupling decreases ATP formation
- ATP consumption > ATP production
- ATP imbalance causes hypermetabolism and metabolic acidosis
- Hypermetabolism stimulates ventilation
- Metabolic acidosis is balanced by respiratory alkalosis
- The patient is sedated and paralyzed, stops ventilating
- Acidosis worsens, pH goes down
- Salicylate rapidly moves into the brain and heart
- You fill out another death report
In animal studies, small decreases in serum pH cause big increases in brain aspirin concentrations.12
So, what do you do if you need to intubate a sick aspirin patient? First, don’t intubate them. But, if you must, my recommendation is to push bicarb (1-2 mEq/kg), use rapid sequence induction, and hyperventilate (rate and depth) when you bag them, intubate them (hopefully in one quick pass), push more bicarb, set the ventilation rate higher than normal, and keep your bicarb drip running. As for the vent settings, I am not going to pretend I know anything about ventilators – consult your intensivist. But, consider high respiratory rates in the beginning with a goal of getting PCO2 < 20 mmHg until you can get serum pH under control with bicarb.13 It should also be evident now that if you are intubating a salicylate overdose you need to closely monitor pH. These patients need repeated checks of arterial pH (not a bad indication for an arterial line). And after you have intubated a bad salicylate overdose, you should start pacing nervously while you check your watch asking “when is renal going to get here?” And, although I haven’t mentioned it (and a topic worthy of its own post), if you have a bad salicylate overdose they need emergent hemodialysis.
For an acute overdose, dialysis is often recommended for any of the following:
- A salicylate level >100 mg/dl
- Altered mental status
- Evidence of pulmonary edema
So next time you have an unconscious uncoupling salicylate overdose, don’t think of Gwyneth, think of bicarb and breathing.
Hello
Thank you for this excellent piece.
In an event you have to intubate a massive aspirin overdose (if you must), hyperventilation will essentially fail to maintain the pH you want. In setting of pulmonary edema it will even be more difficult and chances are the heart will go into asystole.
The only solution I have is to give a huge slug of NaHcO3 like 200 meQ just before intubation to drive the pH high and maintain on straight drug infusion 100-200MEQ/hr AND dialyze as quick as you can.
Farrukh Mirza MD
Attending Physician
Medical Director ECMO program
Pediatric Critical Care Medicine
Loma Linda University Children’s Hospital