
Arsine (AsH3) – From Reconnaissance Balloons to Integrated Circuits
Steven Curry, M.D.*
George Warpinski, M.D.*
Eugene Ngai, M.S.#
* University of Arizona College of Medicine – Phoenix
Department of Medical Toxicology
Banner – University Medical Center Phoenix
Phoenix, AZ
Introduction
The famous 19th century scientist, John Tyndall, reported that inhalation of hydrogen gas produced a voice “so weakened as to become a mere squeak.”

In the pre-helium era, then, chemists and physicists would intentionally inhale hydrogen produced by mixing zinc with sulfuric acid for scientific demonstration and entertainment. In 1841, Mr. Brittan, a chemist and druggist, inhaled on two occasions, about 150 in3 (~2.5 liters) of hydrogen generated from zinc and sulfuric acid “for the purposes of producing the shrill voice of Tyndall’s experiment.” He immediately became ill after the second inhalation and died six days later. It was not a good death. His illness comprised severe vomiting, oliguria with bloody urine, extremity pain/paresthesias, flank pain, weakness, and a greenish-yellow skin hue. Both the zinc and sulfuric acid were found to contain significant amounts of arsenic. His death resulted from inhalation of arsine (AsH3), the hydride of arsenic, also known as arseniuretted hydrogen gas.
Medical toxicology fellows should become familiar with various metal hydride gases. Some metal hydrides are extensively used in semiconductor and other electronics manufacturing, which has a major footprint in Arizona where we encounter them. But these and other metal hydrides are used for other purposes, as well. While I won’t discuss all of the metal hydrides in Fellow Friday posts, I will address some major ones.

One of our fellows, Dr. George Warpinski, joins me as we begin with arsine, the hydride of arsenic. I also want to welcome and express gratitude to Eugene Ngai, who is internationally recognized for his expertise in the manufacture, safe use, and storage/transport of specialty gases. Eugene and I have shared metal hydride war stories, and I wish I could absorb just a fraction of his knowledge and experience. He began manufacturing arsine and phosphine in 1972 and now leads ChemicallySpeakingLLC, a company that provides consultative and educational services regarding metal hydrides and other gases.
Since this is our first post on hydrides, we will first briefly review how some metal hydrides are used in semiconductor manufacturing while covering some very general properties of these gases and their storage. We then will direct our attention to arsine, emphasizing how arsine is encountered in occupational or other settings. While most arsine is used to manufacture semiconductors, LEDs, lasers and satellite solar panels, we’ll see that, historically, most arsine poisonings have had nothing to do with these industries. As always, we will be bouncing around between related side-topics, including death from wallpaper, biomethylation, submarines, aerial balloons, and raining sand – the fun and eclectic nature of medical toxicology.
Metal hydrides and Semiconductor Manufacturing
We are going to very briefly touch on two major processes used in semiconductor/electronics manufacturing: metal organic chemical vapor deposition (MOCVD), and ion implantation.
MOCVD
The manufacture of various semiconductors or other electronic devices requires depositing thin films of monocrystalline or polycrystalline layers containing arsenic or phosphorus mixed with metals such as indium, gallium, aluminum or boron. The crystalline layer is deposited onto silicon or gallium arsenide substrates (wafers) using MOCVD. The metal hydride (or another gaseous form of the metal) and a metal organic vapor (e.g., trimethylgallium, trimethylindium) are introduced into a reaction chamber in the MOCVD tool containing wafers, which are heated. Both the metal hydride and metal organic vapor will react on the hot wafer surface to form a crystalline electrically active layer. The longer the reaction runs (or higher temperature, etc.), the thicker the layer deposited.

The largest use of arsine or phosphine is for manufacture of light-emitting diodes (LEDs), lasers, and photovoltaic cells. Cylinders (tanks) of metal hydride gases used for MOCVD usually reside in a metal gas cabinet or dedicated gas room outside the clean room where MOCVD takes place, and the gases are piped into the MOCVD unit. Typically these facilities reside on a single floor. Relatively large, portable compressed gas cylinders, similar to the large oxygen cylinders we encounter in hospitals, are used.

Right: Arsine and silane 49 L cylinders in gas cabinets in a GaAs manufacturing facility.
Of note, various types of chemical vapor deposition other than MOCVD are used in semiconductor processing. We are touching on MOCVD simply as an example of a common process in which metal hydride gases are used.
Ion implantation
The more commonly manufactured semiconductor device is an integrated circuit (IC) that contains multiple (sometimes > 1 billion) transistors, capacitors, and resistors tied together on one chip. To make these chips, multiple tools are used with different chemicals to deposit, etch, and mask, etc. One step involves “doping” substrates with a semiconducting metal in a process known as ion implantation. The metal is introduced as a gas (hydride or other), ionized, and then accelerated before impacting into a microscopic or larger area of a target (e.g., silicon). In the example below, arsenic is implanted into a wafer using arsine. Phosphine could be used to deposit phosphorus, diborane (or another boron gas) to deposit boron, etc.


Only trace amounts of arsine or phosphine are required for ion implantation, so cylinders are far smaller (2 liter) than for MOCVD (49 liter). The ionization process requires that the cylinders be part of the circuit; therefore cylinders reside in the tool, itself.

I have drawn a very simplified schematic (Figure 8) illustrating where metal hydride cylinders may reside when used for semiconductor manufacturing. Gas cylinder cabinets as well as MOCVD and ion implantation tools are actively exhausted to scrubbers, where waste or accidentally released hydrides are knocked down with aqueous sprays.

For some hydrides, cylinders are available in which the metal hydride is adsorbed onto beads or complexed with an ionic liquid within a cylinder at sub-atmospheric pressure. Not only must the cylinder’s valve be opened, but negative pressure must pull the hydride out of the tank and into the instrument. Other hydrides sometimes reside in pressurized cylinders with special valves so that gas will only flow when the valve on the tank has been opened and negative pressure is applied. When they can be used, these and other systems provide a significant element of safety, given the severe toxicity that can result from inhalation of some of these agents, apart from fire and explosion hazard.
That is, all metal hydrides are quite flammable, with considerably wider flammable ranges than with hydrocarbons such as methane or propane. To emphasize this point, let’s look at silane, the only metal hydride gas that is pyrophoric (autoignition temperature < 130oF in air at 1 atm), but not significantly toxic. (Diborane and phosphine are pyrophoric and quite toxic.)

The fact that silane can sometimes be released into air without immediate ignition, but then suddenly explode, has explained numerous severe and fatal accidents. Silane explosions and fires have resulted in more than 200 significant incidents and 18 deaths in semiconductor/electronics manufacturing plants or laboratories. When silane burns it forms silicon dioxide (sand) submicron-sized particles that are amorphous, not crystalline. Visible sand around a valve of a silane cylinder is evidence that the valve is leaking and requires replacement.

We have taken our fellows in the past to observe a silane burn, in which silane is released into a barrel of water and then out into open air. Check out this video for an example of a similar burn. That tan “smoke” is partially oxidized silicon oxide – it becomes white when fully oxidized.
Arsine is stable enough in air that it can be inhaled, though it still is extremely flammable and potentially explosive (and reactive). Note two warning labels on this large arsine cylinder.

Arsine inhalation
Most medical toxicology fellows associate inhalation of arsine with hemolysis and renal failure. Let’s quickly review the most common signs and symptoms of arsine toxicity following the typical arsine exposure to be sure we are all on the same page as we begin. Figure 12 summarizes some properties of arsine.

Arsine is a colorless gas with a slight odor of garlic, though chemists tell us that pure arsine has no odor. The garlic odor results from low concentrations of methylated arsine species and other contaminants. Note that the odor threshold exceeds the OSHA permissible exposure limit. Toxic exposures can occur without detection of an abnormal odor. Arsine is more dense than air.
Low-level but potentially fatal exposures commonly result in onset of symptoms from one to a few hours after exposure, and include abdominal pain, vomiting, weakness, and grossly red urine, commonly with flank pain, and sometimes fever. The illness can be mistaken for urinary tract stones, but urinalysis reveals dip-stick positive blood (hemoglobin) with few to no red cells on microscopy.

A CBC shows progressive hemolytic anemia of the oxidant type, with Heinz bodies (on special staining), red cell fragmentation, spherocytes, bite cells, low to modest levels of methemoglobinemia, and red/dark plasma.

Mildly elevated transaminases and paresthesias are common. A copper, orange, or bronze-green skin discoloration typically appears. Death occurs over several days from kidney failure and anemia, if untreated. Those who survive may recover from anemia and kidney failure, but go on to develop typical sequelae of other forms of acute arsenic poisoning, including peripheral neuropathy. Acute inhalation of high arsine concentrations produces sudden collapse and death, sometimes with pulmonary edema. Chronic exposure to arsine can result in an illness that resembles chronic inorganic arsenic poisoning.
Mechanisms of hemolysis and renal failure
We’ll say right up front that it’s unknown how arsine causes hemolysis. Red cell oxidant stress certainly occurs, as evidenced by hemolysis, spherocytes, red cell fragmentation, Heinz bodies, bite cells, and methemoglobinemia. But arsine, itself, is a reducing agent. Theories have postulated various events that occur as arsine undergoes oxidation, including hydrogen peroxide formation, depletion of erythrocytic glutathione, production of superoxide radicals, inhibition of red cell superoxide dismutase, inhibition of erythrocytic Na+/K+-ATPase, covalent binding to hemoglobin, coordination binding to heme at iron’s 6th coordination site, and others; experiments have failed to support these conjectures.1,2 That arsenic remains in red cells in a non-dialyzable form after arsine exposure indicates that it binds to some protein(s), and such binding is required in subsequent oxidant stress and hemolysis. We do know that arsine lyses red cells containing oxyhemoglobin, but cannot lyse cells containing only carboxyhemoglobin. Arsine also causes release of heme from erythrocytes containing oxyhemoglobin, but, again, extensive studies have failed to demonstrate arsenic’s binding to heme or to other globins.
Massive hemolysis, of course, can produce acute kidney injury. Both a glomerulopathy and tubular necrosis have been seen in arsine poisoning, and arsenic, itself, may be a contributor to kidney failure.3 Blood, liver, lungs and kidneys display the highest arsenic burdens in those who die from arsine inhalation.
General Mechanisms of Arsine Formation and/or Exposure
Arsine poisoning was recognized more than 200 years ago and continues today across the world, only occasionally involving semiconductor production. We must understand how these exposures take place, and some historical events will make these mechanisms easier to understand and remember.
Reaction of aqueous arsenic solutions with metals
In 1783, Jacques Alexandre Cesar Charles (1746-1823) and his colleagues constructed the first hydrogen balloon in Paris. The balloon was built by sewing together silk sheets to form the envelope, and then painting the silk with rubber dissolved in turpentine. No problem so far, but where to find the hydrogen to fill the balloon?

Charles created hydrogen by pouring about 550 pounds of sulfuric acid onto about 1100 pounds of scrap iron over two days. As ballooning developed across the world, hydrogen generators for balloons became more sophisticated, and hydrogen was used to fill not only aerial balloons, but also children’s balloons. But the same principle of pouring sulfuric acid on a metal (e.g., iron, zinc) was used. Take a look at the acid/iron hydrogen generators used during the U.S. Civil War.

Photograph by Matthew Brady, June 1, 1862. U.S. Library of Congress.
Unfortunately, poisonings and deaths occurred from arsine inhalation during filling of small and large balloons, or when venting gas from aerial balloons after descent, mainly in Europe. Various metals, when mixed with arsenic in aqueous solutions (acidic or alkaline), reduce oxidized arsenic species to arsine. Iron was and still is frequently contaminated with arsenic, and sulfuric acid used in the 18th and 19th centuries contained arsenic, sometimes lots of it. Tyndall’s experiment, which killed Mr. Brittan, relied on this same method of hydrogen production.
It has been commonly written that protons in an acidic solution react with various metals such as zinc to produce nascent hydrogen (H2), which, in turn, reduces oxidized arsenic compounds to arsine. The reaction claimed responsible for reduction of arsenic most commonly is shown as:
3H2 + HAsO2 → AsH3 + 2H20
I heard an excellent lecture by a health physicist in 1988 who pointed out the aforementioned reaction was energetically impossible, and such a reaction would actually run spontaneously in reverse. What really happens is that acid or alkali (or physical grinding) corrodes or removes the superficial metal oxide surface from various metals. If the aqueous solution contains oxidized arsenic compounds such as arsenic oxide, the exposed unoxidized metal will then react with protons (not nascent hydrogen) to catalyze reduction of arsenic to arsine at acidic or alkaline pH values, though the reaction may take place faster at lower pHs, given higher proton concentrations. I was pleased to see this conclusion reiterated in print several years ago by Kilmecki and Carter4, who offered the following equation of what actually occurs, using aluminum metal as an example:
6H+ + 2Al(0) + HAsO2 → AsH3 + 2H2O + 2Al3+
These authors noted that arsine formation is energetically favored whenever various metals react with protons and arsenic in solution, including aluminum, cadmium, iron, lead, nickel, tin and zinc; other metals can do this as well.
Thus, a mechanism of arsine generation and subsequent exposure is when aqueous solutions containing arsenic react with metals.
A few examples of when arsine has been formed in such scenarios are found in Figure 17.5–8

Commercial production of arsine has been described in which acidic solutions containing arsenic species and magnesium or zinc react to form arsine.
Arsenides reacting with water
In 1952, Maccaulay and Stanley reported on six men working with dross from a tin smelting operation who became ill, and two died.9 All experienced hemolysis, hemoglobinuria, and some developed renal failure. Various metal smelting operations result in the formation of dross during purification steps. Dross comprises impurities that float to the top of the molten metal and are skimmed off the surface. Given many metal ores are contaminated with arsenic, the purification of those metals during smelting results in production of dross containing metal arsenides. For example, let’s consider smelting of tin, responsible for the poisoning of these men.

During tin smelting, arsenical impurities float to surface, and dross containing tin arsenide (AsSn) is pulled off. The dross is then taken to another furnace for smelting where the arsenic is oxidized and aluminum is added. Aluminum then combines with the arsenic to form aluminum arsenide (AlAs), which because of its higher melting point, floats to the surface of the molten tin as yet another dross and is skimmed off the top, allowing tin from the original dross to be purified and reclaimed.

Many arsenides (arsenic covalently bound to a less electronegative metal) release arsine gas upon contact with water, and care must be taken to keep arsenides dry during storage, transport, and smelting. Toxicology fellows may recognize the analogy to the release of phosphine (PH3) gas from aluminum, zinc or magnesium phosphide rodenticides when mixed with water. With regard to these six men, they were working with or near AlAs dross that accidentally became wet, resulting in liberation of arsine. In other industrial incidents, 14 workers were poisoned (3 deaths) when moisture reacted with AlAs dross in purification of lead alloys, rather than those of tin.10 In yet another incident, 3 men became ill when water was sprayed for dust control on arsenic-containing slag (containing arsenides).11 One man became ill from breathing arsine generated during a semiconductor product recycling operation in which water reacted with crushed gallium arsenide in a ball mill.12
Thus, a second major means by which arsine is accidentally produced is when arsenides come in contact with water.
Arsine is also manufactured for commercial use by reacting sodium arsenide or zinc arsenide with dilute hydrochloric acid.
Arsine cylinders
Given that arsine is used in various industrial processes, arsine cylinders must be filled, transported, stored, and connected/disconnected to piping for various purposes.

During the fall of 1974, after several days of sailing through rough seas, four crew members entered a hold of their ship – the Asiafreighter – to assess its cargo for damage.13 Several hours later the men began to fall ill, exhibiting myalgias, fever, gastrointestinal symptoms, and hematuria. Four additional crew members fell ill as well, and all eventually ended up at King’s College Hospital for management. On evaluation, each crew member exhibited a degree of anemia due to hemolysis as well as acute kidney injury, with the most severe cases requiring exchange transfusions and hemodialysis. Several other crew members were evaluated at another hospital, but were less effected. A physician had recognized the findings of arsine poisoning, and records confirmed cargo included two 49 liter cylinders of pure arsine in Bay 3. In rough seas, one cylinder valve had partly opened and leaked virtually all contents out through the dust cap and into the ventilation system. This accident led companies to wire the valve handles shut for shipments.

We are aware of numerous incidents, almost none described in the medical literature, in which release of arsine from cylinders, supply lines, or manufacturing tools has resulted in exposures, poisonings and, at times, deaths. Eight examples are provided in figure 22 as illustrations.
Release of preformed arsine from cylinders represents a third means of arsine exposure.

Incident #2 has been described in the literature. **7218060**
In the Phoenix area, our hazmat teams have used various measurement devices over the years to detect metal hydrides in air when responding to incidents. Beginning in the early 1980s they used Dräger Tubes® and Matheson® hydride meters. We currently use HAZMATCAD Plus®, which can detect arsine, phosphine, diborane and many other toxic agents.

Electrolysis
On 27 May 1911 the HMS D4, a British D class Diesel/electric submarine was launched. Two deployments in 1916 during WWI were accompanied by mass illness among the crew. In May 1916 the D4 was submerged 17 hours daily for 7 days before having to return to base. A trip on June 3rd resulted in so much illness it had to return only 4 days later. Illness also overtook the crew of the HMS D3 on three trips around the same period of time.14

Signs and symptoms in affected men included abdominal pain, vomiting, dyspnea, dark red/brown urine, facial edema, jaundice, albuminuria, and anemia with low erythrocyte concentrations and paresthesias. An astute pathologist, Sheldon Dudley, recognized apparent hemolysis and suspected arsine to be the cause, though a source was not apparent. An investigation led to the discovery that plates in the batteries comprising an antimony-lead alloy were contaminated with 0.2% arsenic. During charging, arsenic leached into the electrolyte solution and moved to the batteries’ negative plates where it was reduced to arsine, which then entered the submarine’s atmosphere. Replacement of batteries led to prevention of additional illness.
Thus, a fourth method of unwanted arsine production or release is when electrolysis reduces arsenic to arsine.
Electrocoagulation of waste water containing arsenic and other toxic metals is sometimes used in semiconductor, mining, and other industries to remove metals such as arsenic before discharge into sewers or the environment. Electrocoagulation involves electrolysis with potential for arsine generation.15,16 Electrolysis is also used commercially to manufacture arsine. In fact, some semiconductor plants use on-site arsine generators that create arsine with electrolysis in sealed units under sub-atmospheric pressure, on-demand, for ion implantation. An example of one system is shown in Figure 25.
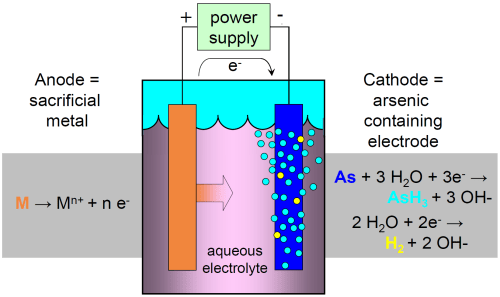
Treatment of Arsine Poisoning
We will only briefly mention treatment of acute arsine poisoning. Exchange transfusions have long been advocated, both to remove arsenic (much of which is in red cells, at least initially) and to correct anemia. More recently plasmapheresis has been used to remove hemoglobin and other constituents, including arsenic, and make room for transfusions.17–20

While treatment with chelating agents, including BAL, does lessen hemolysis and mortality in animals poisoned with arsine, the use of chelators to treat humans with arsine poisoning has not gained acceptance, from the standpoint of lessening hemolysis.21,22 But there are no observational or controlled human trials to guide us. We advocate chelation with BAL or with DMSA (if not in kidney failure) for the purpose of hopefully lessening later sequelae, such as peripheral neuropathy and bone marrow depression. If hemolysis is lessened, that is an extra benefit.
Differential Diagnosis
The diagnosis of arsine poisoning is most easily made by recognizing a scenario for exposure along with characteristic clinical findings. Urine and blood arsenic concentrations will be elevated.23 But what other substances might produce acute oxidant hemolytic anemia with renal failure following acute inhalation?
Stibine
Stibine, the hydride of antimony, is most often listed first in the differential diagnosis of arsine toxicity. Stibine has an “unpleasant odor” similar to hydrogen sulfide. The association of stibine and hemolysis dates back to 1904, when Stock and Guttmann published their paper on stibine and yellow antimony.24

Stibine bubbled through a suspension of erythrocytes behaved like arsine, causing hemolysis and formation of some methemoglobin. With regard to mice, they found inhalation of 1% stibine (10,000 ppm) produced death in seconds; 0.1% stibine produced death in less than one minute; and inhalation of 0.01% stibine produced death after 101 minutes. (Both stibine and arsine kill almost instantly when inhaled at high concentrations, and this appears unrelated to hemolysis – the mechanisms of lethality are unknown, but presumably involve neuro- and/or cardiovascular toxicity.) The authors accidentally sniffed some stibine and experienced dizziness, headaches and nausea. In 1946 Webster generally described experiments in which dogs and cats inhaled 40 to 45 ppm stibine for 1 hour, with death subsequently occurring over hours to a day.25 However, he noted hemoconcentration without hemoglobinuria. On the other hand, guinea pigs inhaling 65 ppm for an hour developed hemoglobinuria and anemia over a few days before recovery.
Despite the findings of Stock and Guttmann, and of Webster, the medical literature is devoid of documented stibine poisoning in human beings, except for a single report of three workers exposed to stibine, arsine and hydrogen sulfide simultaneously as water was added to dross containing arsenic, antimony and sulfur.26 Two of the men experienced weakness, headache, abdominal and lumbar pain and hematuria. It is unknown whether stibine played any role in their illnesses. The lack of reports of stibine toxicity may, in part, represent its thermodynamic instability in air and in storage compared to arsine. In fact, stibine is so unstable that it usually isn’t available in cylinders for commercial or research use. If stibine inhalation ever produces hemolysis in humans, it must be an extremely rare event.
Germanium hydrides
Use of germane and digermane has dramatically increased since development of silicon germanium for transistor semiconductor junctions. These gases have a “pungent odor”, though Eugene tells me germane smells like arsine to him. Arsine and phosphine both smell strongly of garlic to me, so I assume germane may smell like garlic to many persons.

Germane is commonly listed as a hemolytic poison with claims of toxicity similar to that of arsine, but is this true? We start with a frequently-cited 1924 paper by Paneth and Joachimoglu, who described 5 experiments in which a total of three mice, one frog, one rabbit, and two guinea pigs inhaled between 100 and 2170 ppm germane for between 30 minutes and 1 hour.27 The two guinea pigs displayed what the authors called hemoglobinuria, but we don’t have results of a microscopic examination of blood or urine or histology of the kidneys and urinary tract. No measurements of blood hemoglobin or red cells were reported. In a subsequent in vitro experiment, the authors specifically stated they failed to produce hemolysis with germane. We don’t see much to support hemolysis from this report.
Ivanov and colleagues exposed rats to 22.3 ppm GeH4 for 4 hours daily for 30 days.28 There were no detectable differences from controls during the first days of the experiment, indicating no changes from acute exposures. After two weeks some hemosiderin staining was noted in livers and spleen, but red cells actually became more resistant to osmotic hemolysis and RBC counts and hemoglobin concentrations increased – the opposite of what would be expected with hemolysis.
In 1974, Gus’kova studied germane inhalation in mice, rats and guinea pigs.29 Death occurred over 1-2 days in 50% of mice acutely inhaling ~440 ppm GeH4 for 2 hours.30 Deaths were characterized by uncoordinated movement and convulsions and coma with injury to the liver and kidneys; no hemolysis was reported following acute exposures. Gus’kova also found that mice and guinea pigs inhaling about 78 ppm germane for a full month had a minimal drop in hemoglobin concentration (mean 1.3 g/dL decline), but rats breathing about 78 ppm for 2 months actually experienced increased hemoglobin and red cell concentrations without rises in reticulocyte counts.29 Thus, neither acute nor chronic germane exposures produced significant hemolysis.
Gus’kova and Babina (1984) reported that workers inhaling germane experienced headache, vertigo, weakness and collapse.30 No specific description of hemolysis or hemoglobinuria was described.
Despite claims of producing toxicity similar to arsine, germane does not appear to be a hemolytic toxin. But germane is available in cylinders so that large quantities could be released into a confined space. The use of digermane continues to become more popular, and there are no reports of hemolysis in animals or humans with that hydride, either.

Hydrogen telluride
Hydrogen telluride has a garlic or “rotten leeks” odor and is so unstable that it also is typically unavailable in cylinders for industrial use. The semiconductor industry usually uses diethyltelluride, instead. Webster is sometimes cited for describing hemolysis in guinea pigs inhaling high concentrations of H2Te, but his paper actually stated in a single sentence that H2Te mixed with heparinized guinea pig blood in vitro produced what he called spline cells.25 No hemolysis in animals or humans were reported, and none have since appeared.
Hydrogen selenide
Hydrogen selenide smells of decayed horseradish and is primarily used to grow zinc selenide lenses for infrared detectors. The gas is quite irritating upon inhalation and quickly forms selenious acid when encountering moisture in or upon us, producing a garlic odor. Hydrogen selenide mixed in vitro with guinea pig blood also produced deformed red cells, but did not produce hemoglobinuria.25 There exist no reports of H2Se producing hemolysis in animals or humans. Inhalation toxicity appears mainly to be irritation.
From the standpoint of acute inhalation, then, it is difficult to imagine a likely cause of massive hemolysis and renal failure other than arsine. A list of ingested or injected substances capable of causing severe oxidant hemolysis with some methemoglobinemia is long and can’t be discussed here. Some were mentioned in a previous post on methylene blue infusions for methemoglobinemia. We’ll simply note naphthalene, chlorates, chromates, dapsone, aniline and nitrobenzene as examples. We also don’t have space here to address workers who may have severe glucose-6-phosphate dehydrogenase deficiency and implications for exposure to industrial chemicals.
Wallpaper, Arsenic, and Not Arsine
I own a wonderful book by Lucinda Hawksley titled Bitten By Witch Fever. Wallpaper & Arsenic in the Victorian Home. Pigments used in wallpaper and plaster in the 1800s commonly contained copper arsenite, known as Scheele’s Green. The book contains samples of wallpaper reproductions (without arsenic), and also provides a great history on the entire arsenic & wallpaper era. In the 1800s there arose great concern that dust and fragments of plaster and wallpaper containing copper arsenite that fell onto floors, tables, beds and elsewhere contaminated homes and produced illness and death from arsenic poisoning.

Apart from arsenic dust and debris, it also became widely accepted in Europe at the time that mold on wallpaper, living off of damp cellulose and wallpaper paste, converted arsenic to arsine or some other gas with a garlic odor. The gas became known as Gosio Gas, named after Bartolomeo Gosio, who demonstrated that certain fungi could convert inorganic arsenic compounds to a volatile form. If the garlic (or “mousey”) odor of Gosio Gas was detected in a home where an unexplained death occurred, the gas was to blame. Arsine was often named, though other volatile arsenic compounds were suspected. The famous chemist, Leopoid Gmelin, suspected dimethylarsinoxide. Cullen and Bently provide a superb and entertaining review on how Gosio Gas was eventually identified as trimethylarsine, a gas of extremely low toxicity (animal 4-hour LC50 ~ 20,000 ppm).31

Looking back, there is no convincing evidence that arsenic in wall paper or other decorative items definitely was responsible for illness or death, whether from copper arsenite or trimethylarsine.31 Unrelated to wallpaper, though, Clare Booth Luce, the US ambassador to Italy, developed arsenic poisoning from white lead arsenate that fell from rosettes on her bedroom’s decorated ceiling in the US embassy due to a vibrating washing machine on the floor above. She resigned because of illness in 1956.31,32
Metal Biomethylation and Garlic Odor
When some elements are methylated, they acquire a strong garlic odor. Take a look at a section of the periodic table in Figure 32.

Odors of arsenic, phosphorus, sulfur, selenium, and tellurium range from none to slight, as do many of their inorganic oxides and salts. However, when they become alkylated (e.g., methylated) or incorporated into other organic compounds, they acquire a strong garlic odor. Substituted arsines include monomethyl-, dimethyl-, and trimethylarsine, with strong garlic odors. Recall earlier we noted that methylated contaminants provide arsine with its characteristic odor. Inorganic phosphorus compounds possess a slight odor, but organophosphate insecticides are quite garlicky. Alkylate sulfur to dimethylsulfoxide (DMSO) and watch out!
Microorganisms methylate many metals, and some can take inorganic arsenic all the way to trimethylarsine. How about human beings? What metals can we methylate? When humans methylate metals, it appears to be for purposes of detoxification, as methylated species we form are less toxic than their inorganic counterparts. We can methylate arsenic all the way to dimethyl pentavalent arsenic, but not to trimethylarsine, which is even much less toxic. Humans also can take inorganic selenium compounds to dimethylselenide, and tellurium compounds to dimethyltelluride, both of which display strong garlic odors. A man or woman may ingest excessive selenium supplements with only weak garlic odors, develop chronic selenium toxicity, and then present with a strong garlic odor from conversion of selenium to monomethyl- and dimethylselenide. We have cared for children who ingested inorganic tellurium salts (used for metal darkening) who noticeably smelled of garlic for over a year (dimethyltelluride).33 While there is some evidence we can also methylate bismuth, the garlic odor noticed on the breath of bismuth miners (bismuth breath) or of patients who ingested bismuth oxynitrate for medicinal purposes was actually due to tellurium also found in the mines and medication. Humans don’t methylate antimony.
Just to remind us of the complexity of nature, there are also soil microorganisms that reduce inorganic arsenic to arsine.34 At one time there was concern that sudden infant death syndrome resulted from bacterial formation of arsine in pillows or other bedding used in cribs. Stibine was suspected as well. No support was found for any of this.
Conclusion
We are left with four main scenarios in which arsine gas is produced and/or encountered:
- aqueous solutions of oxidized arsenic compounds in contact with metals
- metal arsenides in contact with water/moisture
- release of arsine from cylinders
- electrolysis in which solutions contain arsenic
While semiconductor and electronics industries are responsible for consuming the great majority of the world’s manufactured arsine, exposures to arsine released from cylinders or associated devices have not been the most common scenarios in which serious arsine poisoning has occurred. At least, to date.
In the meantime, be careful when filling your hydrogen balloons, keep those metal arsenides dry, take a respirator when cruising on WWI submarines, and don’t substitute home-made hydrogen for helium when you want to sound like a chipmunk to your children.
Post Script
Medical toxicology fellows, here are a few questions to contemplate, research and discuss with faculty members.

- James Marsh (1794–1846) developed a forensic test for detecting arsenic, known as the Marsh test or Marsh reaction. How did this test work, and how was arsine generation involved? Why was hydrogen sulfide usually bubbled through the acid (usually sulfuric) when fresh reagents were prepared for the test? Finally, what other metals could produce an initial false positive result for arsenic?
- While human methylation of inorganic arsenic species to monomethyl and dimethyl compounds detoxifies the metal with regard to what is traditionally considered arsenic poisoning, is this true for genotoxicity? What arsenic compound is the most damaging to DNA and is suspected to be the most important carcinogenic species? What cancers are associated with chronic arsenic exposure?
- Human beings don’t alkylate inorganic lead, mercury, or tin. What can we say about the toxicity of alkyllead, alkylmercury, and alkyltin as compared to their inorganic forms, and what toxicities are associated with those organometals? Why might neurotoxicity be such an issue?
- 1.Rael L, Ayala-Fierro F, Bar-Or R, Carter D, Barber D. Interaction of arsine with hemoglobin in arsine-induced hemolysis. Toxicol Sci. 2006;90(1):142-148. doi:10.1093/toxsci/kfj054
- 2.Hatlelid K, Carter D. Reactive oxygen species do not cause arsine-induced hemoglobin damage. J Toxicol Environ Health. 1997;50(5):463-474. doi:10.1080/00984109708984002
- 3.Muehrcke R, Pirani C. Arsine-induced anuria. A correlative clinicopathological study with electron microscopic observations. Ann Intern Med. 1968;68(4):853-866. doi:10.7326/0003-4819-68-4-853
- 4.Klimecki W, Carter D. Arsine toxicity: chemical and mechanistic implications. J Toxicol Environ Health. 1995;46(4):399-409. doi:10.1080/15287399509532045
- 5.Parish G, Glass R, Kimbrough R. Acute arsine posioning in two workers cleaning a clogged drain. Arch Environ Health. 1979;34(4):224-227. doi:10.1080/00039896.1979.10667403
- 6.DePalma A. Arsine intoxication in a chemical plant. Report of three cases. J Occup Med. 1969;11(11):582-587. https://www.ncbi.nlm.nih.gov/pubmed/5348636
- 7.Risk M, Fuortes L. Chronic arsenicalism suspected from arsine exposure: a case report and literature review. Vet Hum Toxicol. 1991;33(6):590-595. https://www.ncbi.nlm.nih.gov/pubmed/1808840
- 8.Pakulska D, Czerczak S. Hazardous effects of arsine: a short review. Int J Occup Med Environ Health. 2006;19(1):36-44. doi:10.2478/v10001-006-0003-z
- 9.MACAULAY D, STANLEY D. Arsine poisoning. Br J Ind Med. 1956;13(3):217-221. doi:10.1136/oem.13.3.217
- 10.Arsine poisoning in metal refining plant. Fourteen simultaneous cases. Acta Med Scand Suppl. 1968;496:7-8. https://www.ncbi.nlm.nih.gov/pubmed/5259510
- 11.KIPLING M, FOTHERGILL R. ARSINE POISONING IN A SLAG-WASHING PLANT. Br J Ind Med. 1964;21:74-77. doi:10.1136/oem.21.1.74
- 12.Yoshimura Y, Endo Y, Shimoda Y, Yamanaka K, Endo G. Acute arsine poisoning confirmed by speciation analysis of arsenic compounds in the plasma and urine by HPLC-ICP-MS. J Occup Health. 2011;53(1):45-49. doi:10.1539/joh.l10108
- 13.Wilkinson S, McHugh P, Horsley S, et al. Arsine toxicity aboard the Asiafreighter. Br Med J. 1975;3(5983):559-563. doi:10.1136/bmj.3.5983.559
- 14.Dudley S. Toxemic anemia from arseniuretted hydrogen gas in submarines. J Indus Hyg. 1919;1:215-245. https://www.google.com/books/edition/The_Journal_of_Industrial_Hygiene/-fQ-AQAAIAAJ?hl=en&gbpv=1&dq=toxemic+anemia+from+arseniuretted+hydrogen&pg=PA215&printsec=frontcover
- 15.Accident: 201954104 – Four Employees Injured When Exposed To Arsine Gas. Occupational Safety and Health Administration (OSHA). Accessed May 21, 2021. https://www.osha.gov/pls/imis/accidentsearch.accident_detail?id=201954104
- 16.Basha CA, Selvi SJ, Ramasamy E, Chellammal S. Removal of arsenic and sulphate from the copper smelting industrial effluent. Chemical Engineering Journal. Published online July 2008:89-98. doi:10.1016/j.cej.2007.10.027
- 17.Danielson C, Houseworth J, Skipworth E, Smith D, McCarthy L, Nanagas K. Arsine toxicity treated with red blood cell and plasma exchanges. Transfusion. 2006;46(9):1576-1579. doi:10.1111/j.1537-2995.2006.00931.x
- 18.Song Y, Wang D, Li H, Hao F, Ma J, Xia Y. Severe acute arsine poisoning treated by plasma exchange. Clin Toxicol (Phila). 2007;45(6):721-727. doi:10.1080/15563650701502675
- 19.McCarthy L, Danielson C, Houseworth J, et al. Transfusion medicine illustrated. Black plasma resulting from inhalation of arsine gas. Transfusion. 2006;46(8):1267. doi:10.1111/j.1537-2995.2006.00890.x
- 20.Huang C, Zhang P, Liu H, et al. Treatment for acute arsine poisoning with blood purification. Intensive Care Med. 2015;41(3):549-550. doi:10.1007/s00134-014-3595-y
- 21.LEVVY G. A study of arsine poisoning. Q J Exp Physiol Cogn Med Sci. 1947;34(1):47-67. doi:10.1113/expphysiol.1947.sp000915
- 22.KENSLER C, ABELS J, RHOADS C. Arsine poisoning, mode of action and treatment. J Pharmacol Exp Ther. 1946;88(1):99-108. https://www.ncbi.nlm.nih.gov/pubmed/20274531
- 23.Apostoli P, Alessio L, Romeo L, Buchet J, Leone R. Metabolism of arsenic after acute occupational arsine intoxication. J Toxicol Environ Health. 1997;52(4):331-342. doi:10.1080/00984109708984068
- 24.Stock A, Guttmann O. Ueber den Antimonwasserstoff und das gelbe Antimon. Ber Dtsch Chem Ges. Published online January 1904:885-900. doi:10.1002/cber.190403701148
- 25.WEBSTER S. Volatile hydrides of toxicological importance. J Ind Hyg Toxicol. 1946;28:167-182. https://www.ncbi.nlm.nih.gov/pubmed/21000283
- 26.Dernehl CU, Stead FM, Nau CA. Arsine, Stibine, and Hydrogen Sulfide:Accidental Generation in a Metal Refinery. American Industrial Hygiene Association Quarterly. Published online April 1944:361-362. doi:10.1080/00968204409344172
- 27.Paneth F, Joachimoglu G. Uber die pharmakologischen Eigenschaften des Zinnwasserstoffs und Germaniumwasserstoffs. Berd deut Chem Ges. 1924;57:1925-1930.
- 28.Ivanov N, Germanova A, Gus’kova E. [Comparison of the body’s reactions to the repeated action of germanium hydride and tetrachloride]. Gig Tr Prof Zabol. 1976;(1):34-38. https://www.ncbi.nlm.nih.gov/pubmed/950153
- 29.Gus’kova E. [Toxicology of Germanium hydride]. Gig Tr Prof Zabol. 1974;18(2):56-57. https://www.ncbi.nlm.nih.gov/pubmed/4839911
- 30.Gus’kova E, Babina M. Occupational safety and germanium hydride. Bezop Tr Prom. 1984;1:40-44.
- 31.Cullen W. The toxicity of trimethylarsine: an urban myth. J Environ Monit. 2005;7(1):11-15. doi:10.1039/b413752n
- 32.ARSENIC IN THE HOUSE. The Lancet. Published online July 1956:182-183. doi:10.1016/s0140-6736(56)91706-8
- 33.Yarema M, Curry S. Acute tellurium toxicity from ingestion of metal-oxidizing solutions. Pediatrics. 2005;116(2):e319-21. doi:10.1542/peds.2005-0172
- 34.Cheng C, Focht D. Production of arsine and methylarsines in soil and in culture. Appl Environ Microbiol. 1979;38(3):494-498. doi:10.1128/AEM.38.3.494-498.1979
Leave a Reply